Ενδιαφέρον περιστατικό μεγάλης μάζας καρκίνου του πνεύμονα, στο κεντρικό site της Bristol Myers Squibb (BMS) με πλήρη μεταβολική ανταπόκριση χωρίς τη διενέργεια χειρουργείου με τη χρήση νεότερης γενιάς συνδυασμού ανοσοθεραπείας
Written by Dr. Theofanis Floros, MD, Medical Oncologist
Director, 5th Oncology Dept. Metropolitan General Hospital
Assoc. Director, Athens Naval & Veterans Hospital Oncology Dept.
Patient History
• Ε.Β., Male, Caucasian, aged 68 years
• Heavy smoker, works as a fisherman
• During clinical investigation for recurrent left shoulder pain, a large mass in his left upper lobe (LUL) was initially revealed.
• A bronchoscopy was performed and biopsy from an exophytic endobronchial mass
• Pathology report was consistent with squamous cell lung cancer
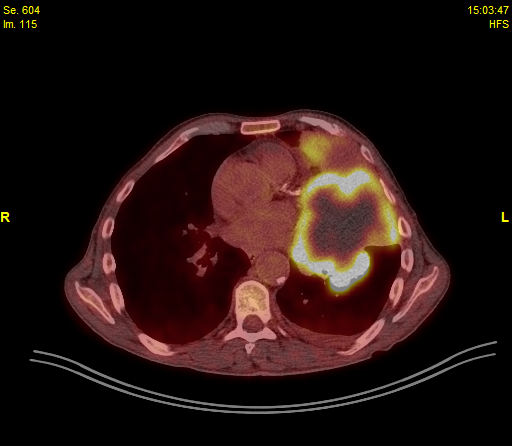
• A subsequent PET/CT identified a cT4 mass (over 10cm) eroding the 3rd left rib, mildly hypermetabolic mediastinal lymph nodes (1L, 3A, 5, 6), left inner mammary LN as well as a small pleural effusion
• Personal history comprised:
a)Current smoker (> 100 pack years)
b)Cannabis user
• ECOG PS: 1 (Restricted in physically strenuous activity)
• Medications: None
• No abnormal extra thoracic findings including CNS.
• No abnormal findings for the clinical examination of the abdomen and both central and peripheral nervous system
• Reduced left respiratory sound during auscultation
• Normal ECG and cardiac U/S
• Appetite decrease and weight loss (>15% over the last 3 months)
Diagnosis
Baseline Clinical Data
• PET/CT: cT4 mass (over 10cm) eroding the 3rd left rib, mildly hypermetabolic mediastinal lymph nodes (1L, 3A, 5, 6), left inner mammary LN, small pleural effusion
• Bronchoscopy biopsy: squamous cell Lung cancer (IHC positive for AE1/3, p40, negative for TTF-1)
• Final Diagnosis: Stage IV (T4N3M1a) sqNSCLC (although no EBUS or pleural effusion aspiration performed)
• PD-L1 testing: not performed
Initial Imaging
Disease Managment
• The patient was fit for 1st line treatment.
• The presence of such bulky disseminated disease and the squamous histology guided clinical decision towards chemoimmunotherapy with combined antiCTLA4/antiPD1 inhibitors as per CheckMate 9LA rationale.
• Treatment started on 5/12/2022 and comprised Ipilimumab 1mg/Kg, Nivolumab 360mg, Paclitaxel 200mg/m2 and Carboplatin AUC5.
• The drug’s tolerance was good and his second “induction” cycle comprising Nivolumab 360mg, Paclitaxel 200mg/m2 and Carboplatin AUC5 was administered on 6/14/2022 with accepted toxicity.
• Subsequently the patient received 4 cycles of Ipilimumab/Nivolumab – Nivolumab alone. The symptoms that led to diagnosis were resolved.
• Re-evaluation consistent with Complete Response
• The patient continued Ipilimumab/Nivolumab maintenance every 3 weeks
• Re-evaluations on 12/20/2022 and 5/3/2023 demonstrate continuous metabolic and radiographic response
• Off note diffuse mildly hypermetabolic mediastinal consistent with “sarcoid-like” immune stimulation
• Last to date follow up (consistent with complete metabolic response after > 1 year on treatment
2nd Evaluation – Respective images
Conclusion
• Patient with metastatic squamous NSCLC; sites of disease intra-thoracic
• No CNS involvement
• Treatment comprised dual Ipilimumab/Nivolumab Immunotherapy with two chemotherapy cycles
• Complete metabolic response with minimal toxicity
• Patient receives Ipilimumab/Nivolumab
• Remains in metabolic complete Response after 4th evaluation (> 1 year after treatment initiation)












