Ενδιαφέρον περιστατικό μεταστατικού μελανώματος, στο κεντρικό site της Bristol Myers Squibb (BMS) με εγκεφαλικές μεταστάσεις θεραπευθέν χωρίς τη χρήση κλασσικών μεθόδων όπως η ακτινοβόληση εγκεφάλου
Written by Dr. Theofanis Floros, MD, Medical Oncologist
Director, 5th Oncology Dept. Metropolitan General Hospital
Assoc. Director, Athens Naval & Veterans Hospital Oncology Dept.
Patient History
• C.T., Male, Caucasian, aged 78 years
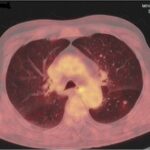
• During a preventive annual thoracic CT for smoking habit, mediastinal lymphadenopathy and a Left Lower Lobe lesion consistent with metastasis was revealed
• Subsequent PET/CT for CUP was ordered with no other hypermetabolic sites identified
• Personal history comprised:
ㅤㅤa) Thyroidectomy due to multinodular goiter
ㅤㅤb) Prostate Benign Hyperplasia (BPH)
ㅤㅤc) Psychotic Depression
• Medications:
ㅤㅤa) Thyrohormone 0.1 mg tb/daily
ㅤㅤb) Dutasteride/Tamsulosine (0.5mg+0.4mg)/daily
ㅤㅤc) Escitalopram 20mg/Olanzapine 5mg/Bromazepam 3mg (each daily)
• ECOG PS: 0
• No pathologic findings for the clinical examination of the thorax, abdomen and both central and peripheral nervous system
• Normal ECG and cardiac U/S
• No appetite decrease and weight loss
• Mild Bradypsychia consistent psychiatric treatment
Diagnosis
Baseline Imaging
• PET/CT: enlarged hypermetabolic mediastinal lymph nodes, LLL metastasis, no other finding
• EBUS-TBNB biopsy: malignant melanoma (IHC positive for Vim, SOX10, MART-1, S-100, negative for AE1/AE3, CD45/LCA, CD-138, p40, HSA)
• After further discussion, the patient reported a regressed pigmented lesion on his left back that was removed a year ago with no pathology examination performed
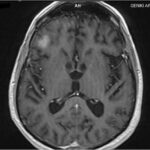
• Brain MRI: 2 Right Temporal Lobe lesions (15.9 & 14.2 mm), 1 Left Frontal Lobe lesion (9.8mm) – no significant perilesional oedema
• Final Diagnosis: Stage IV (TxNxM1d(0)) cutaneous melanoma
• BRAF testing: wild type
Initial Imaging
Disease Managment
• The lack of perilesional brain oedema, CNS symptomatology and the relative small lesion size, omission of either SBRT/WBRT and/or steroid was decided
• Levetiracetam 1gr b.i.d was precautionary prescribed due to temporal lobe lesion presence
• Ipilimumab/Nivolumab as per CheckMate-067 (CM-067) protocol was initiated based on results from CM-067, the phase II CM-204 and the Australian study (NCT02374242) both reported in 2018
• The patient received 4 induction cycles without any severe adverse event (skin rash and grade 2 pyrexia during the first 2 cycles)
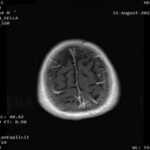
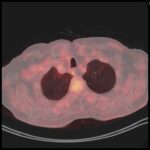
• Re-evaluation consistent with Complete Response both systemic and intracranial
Imaging 1st Evaluation – respective images
Conclusion
• Patient with metastatic cutaneous melanoma; sites of disease both extra and intracranial
• No local CNS treatment
• Dual Ipilimumab/Nivolumab Immunotherapy as per CheckMate-067 administered
• 4 induction cycles – Complete Response with minimal toxicity
• Patient receives maintenance nivolumab
• Remains in Complete Response after 2nd evaluation (January 2023)
• In case of asymptomatic, small size (<30mm) and no need for upfront steroid brain metastasis, omission of Radiotherapy can be pursued
• Radiotherapy can delay systemic immunotherapy initiation and hamper efficacy due to steroid requirement during and after irradiation
• Clinical trials/studies show high rates of intracranial clinical response with ipilimumab/nivolumab immunotherapy combination alone
• This patient’s clinical course consistent with reported data